Abstract
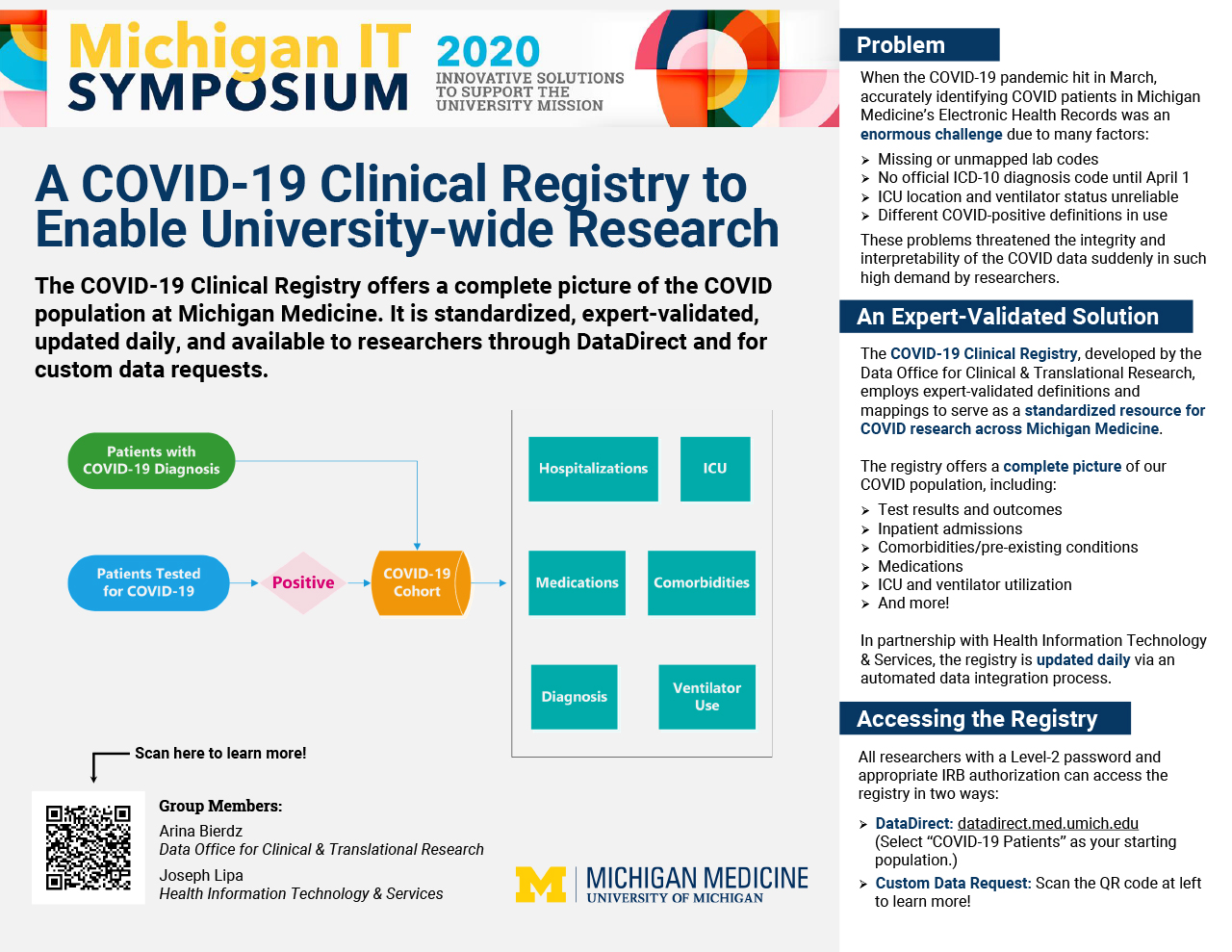
When the COVID-19 pandemic hit in March, accurately identifying COVID patients in Michigan Medicine’s ocean of EHR data presented an enormous challenge. Lab test codes were missing or unmapped, an official ICD-10 diagnosis code had yet to be designated, and different definitions of the COVID-positive population were in use within the U-M research community. In addition, traditional markers such as ICU location and ventilator status became increasingly difficult to capture as the health system expanded critical-care capacity beyond its normal units. These obstacles threatened both the integrity and interpretability of the COVID data suddenly in high demand by researchers across the university.
As a result, the Data Office for Clinical & Translational Research developed the COVID-19 Clinical Registry to serve as a standardized resource for universitywide COVID research. Employing expert-validated definitions and mappings, the COVID-19 Clinical Registry provides a complete picture of the COVID population at Michigan Medicine, including patient-level data on testing, outcomes, inpatient admissions, comorbidities, medications, and ICU and ventilator utilization. In partnership with Health Information Technology & Services, the registry is updated daily via an automated ETL process. Researchers with a Level-2 password and appropriate IRB approval can access the registry via DataDirect or a custom data request.